EC Regulation 1223/2009 has revolutionised cosmetics packaging
What has changed in the cosmetic industry and how companies are to comply with the new rules
11 July, 2013. This date will be remembered in the cosmetics packaging industry as the day on which the EC Cosmetics Regulation 1223/2009came into force.
This new regulation fully replaces Cosmetics Directive 76/768/EEC of 27 July 1976.
Its main purpose is to safeguard consumer’s health through monitoring the information they receive regarding product ingredients and labels.
Which are the new rules for cosmetics manufacturers? What has changed as regards labelling and packaging? ach company is to appoint an internal “responsible person” in charge to supervise that products comply with the Regulation.
The EU Member State where the product is marketed will be responsible for specifying the languages to be used on the labels.
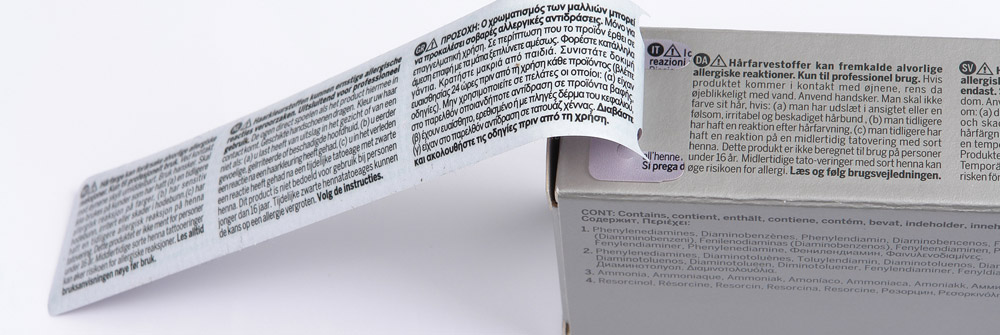
As required by law the packing of cosmetic product must bear, in indelible, easily legible and visible characters, the following information:
- name, corporate name and address of manufacturer or MA holder;
- country of origin where a product is imported into the EU;
- date of minimum durability (Best before date);
- nominal content at the time of packaging, given by weight or by volume;
- the expiry date to use a cosmetic product stored under suitable conditions;
- particular precautions for use, also for cosmetics of professional use;
- batch number, lot code or product reference to permit product identification;
- list of ingredients used to manufacture the product.
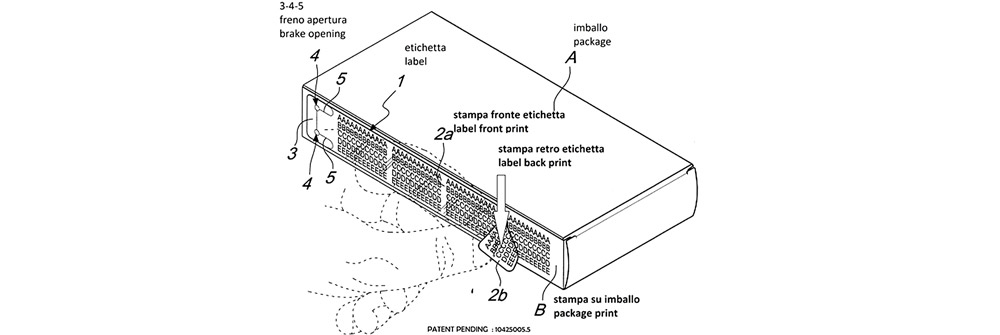
Our F1T3® labelling system meets the requirement of using multilingual labels up to three pages, without the need for expensive multipage adhesives.
Do you want to triple the printing space available for ingredients, information and the like?
Contact an expert online and ask all you want to know about F1T3® or ask for a tailor-made estimate.